| |
Intersurgical has developed, manufactured and supplied innovative respiratory and filtration solutions into the healthcare setting for over 30 years. This expertise has allowed us to design a medical mask specifically for clinical personnel. Utilising our proven mask and filtration technologies, the new i-Pro™ mask is designed to provide comfortable, high quality protection for clinicians working in the clinical environment. |
|
Traditional personal protective masks were originally designed to be used in the industrial environment and are therefore not always suited for the population of healthcare workers, this has resulted in a number of reported issues. The i-Pro™ mask has been designed with clinicians in mind. Using our breathing system filter media, with proven bacterial and viral efficiency in the clinical environment, and a soft, flexible TPE seal, featured in our range of respiratory products, the i-Pro™ mask combines a high level protection with a comfortable and effective fit. |
| |
i-ProTM Features & benefits |
|
|
| |
|
| |
There have been many reports of clinicians with facial sores as a result of wearing PPE masks. The i-Pro™ provides a high quality effective seal whilst being soft and comfortable on the wearer’s face for the duration of use.
i-Pro's high quality seal limits air leakage and eliminates fogging of spectacles.
|
|
 |
| |
i-ProTM product descriptions and codes
 |
| |
|
i-ProTM Videos
| |
i-Pro™ - the principles of personal protection

i-Pro™ fitting guide

|
|
i-Pro™ features and benefits
 |
| |
|
a
Make an enquiry to find out more about our i-ProTM medical mask
References:
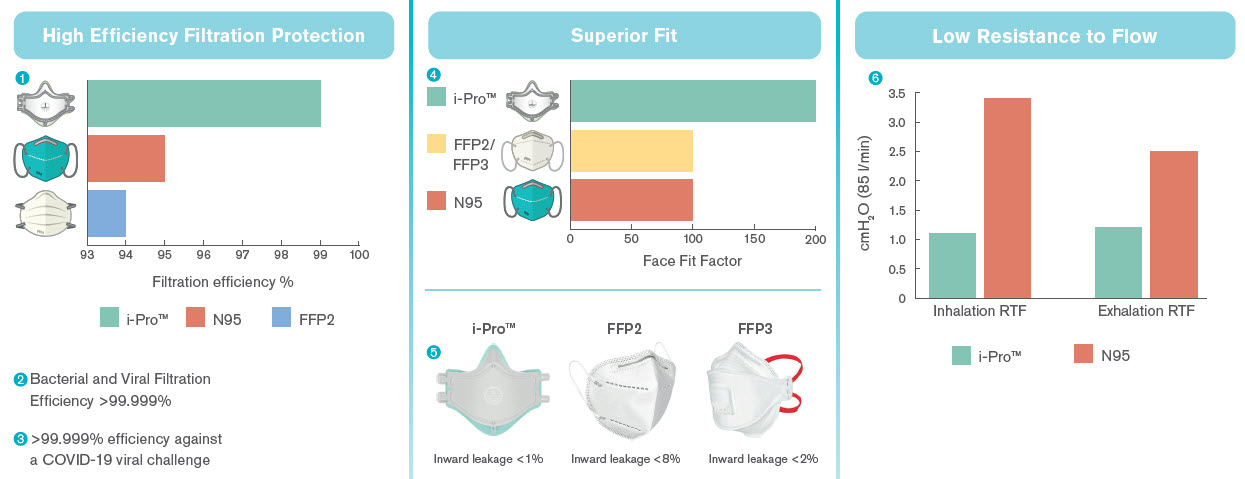
1. i-Pro™ has been independently tested at Nelson Laboratories in USA against the standard 42 CFR PART 84 (N95 standards) and against standard EN 149-clause 7.9.2 (FFP2) has shown to have a filtration efficiency >99%.
2. i-Pro™ has been independently tested at a specialist microbiology laboratory facility against a clinically relevant. Bacterial and Viral challenge that clinicians may see in the clinical setting. A challenge particle is chosen to simulate the size of commonly occurring bacteria/virus. Clinically relevant testing is carried out using Bacillus subtilis (1.0μm x 0.7μm) and Ø174 bacteriophage (0.027μm).
3. The challenge presented in the viral test protocol (Ø174 bacteriophage, 0.027 μm) will be at least as severe as that posed by COVID-19 (0.05 - 0.1μm). As such, it can be concluded that i-Pro™ mask filter media is expected to provide at least the same level of efficiency as reported in the independent microbiology tests when challenged with Coronavirus.
4.A quantative fit test can determine the quality of the seal of a mask on a person’s face with a numerical result, given as “Fit Factor”. The Fit Factor can be measured using a TSI portacount machine. A fit factor is defined as the ratio of substance concentration outside to inside a respirator. A Fit Factor of 100 is required for N95, FFP2 and FFP3 masks.
5 The standard for an FFP mask allows up to an 8% inward leakage for FFP2 masks and a 2% inward leakage for FFP3 masks. This means with an FFP2 mask, potentially 8% of the air breathed in will not be effectively filtered. Independent testing has shown the i-Pro™ to have <1% inward leakage, meaning >99% all of the air you breathe, will be filtered.
6.It has been tested that i-Pro™ has lower inhalation and exhalation resistance to flow than what required by the N95 standards.



